Profile
MINORU.UEDA
Medical Researcher/Maxillofacial Surgeon
MINORU.UEDA
Medical Researcher/Maxillofacial Surgeon 「Times They are a-changin'」
Even with today's advanced medical care, there are still many diseases where patient satisfaction with treatment is low and there is room for improvement. These include cases in which there is no cure for the disease and the patient does not feel that the treatment is sufficiently effective, or cases in which the high cost of treatment places a heavy burden on the patient. Such requests for treatment methods from the patient's point of view are "Unmet medical needs. Therefore, diseases with strong "Unmet medical needs" are the ones that we, medical researcher should place the highest priority on.
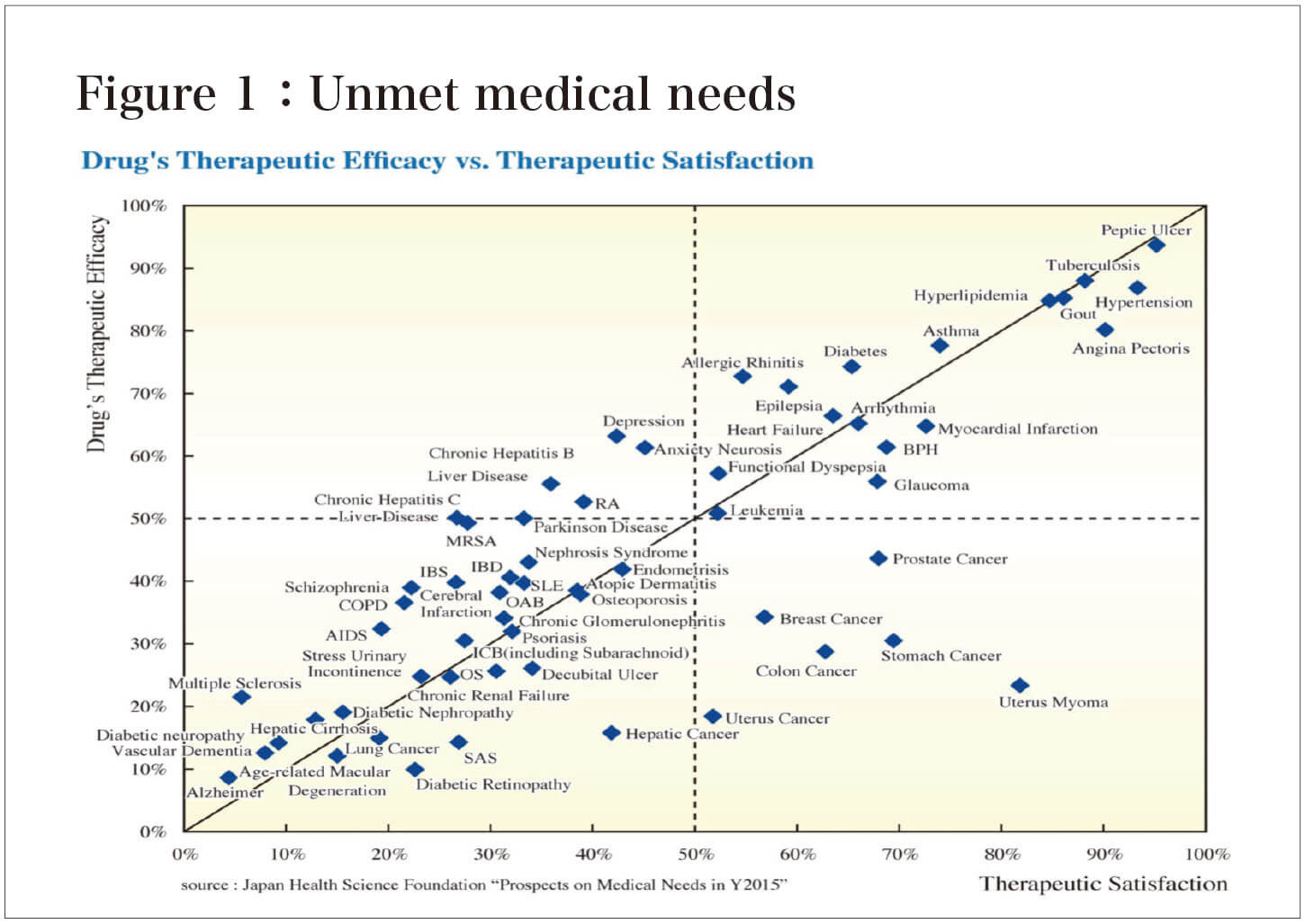
According to the American Association of Research Pharmaceutical Manufacturers, Alzheimer's disease (AD) is a prime example of an "Unmet medical need," with the lowest level of patient satisfaction, indicating that current treatments are of little use (Figure 1)。
AD is a disease with a large number of patients and is likely to increase in the future, and its control is becoming an urgent global issue because of the social and economic pressures it places on society. The direct impact of the increase in AD is a dramatic increase in the cost of medical care and nursing care. However, an even more serious impact is that families (often women and young people) are forced to give up work in order to care for AD patients. For the individual, this will mean a setback in life, and for society, it will directly lead to a shortage in the workforce, which will eventually impede the economic growth of our country. Conversely, if a cure for AD were found, it would not only lead to medical success in restoring the health of the individual patient, but also to social benefits such as an increase in the working population and labor productibility .
As a greeting to you , I would like to first explain the social and economic problems of AD, and then introduce the challenges of current AD treatment and the "Conditioned medium therapy" that may break through these challenges.
The International Alzheimer's Association estimates that the number of patients with dementia (AD accounts for more than 70% of the patients) worldwide will be 76 million in 2030 , respectively. It is estimated that the number of people will reach 135 million in 2050. Data for Japan is available in the White Paper on Aging Society published by the Cabinet Office in 2017. According to the report, it is estimated that 7.3 million people will have AD by 2025 and more than 10 million people by 2050, or one out of every four people over 65 years old.
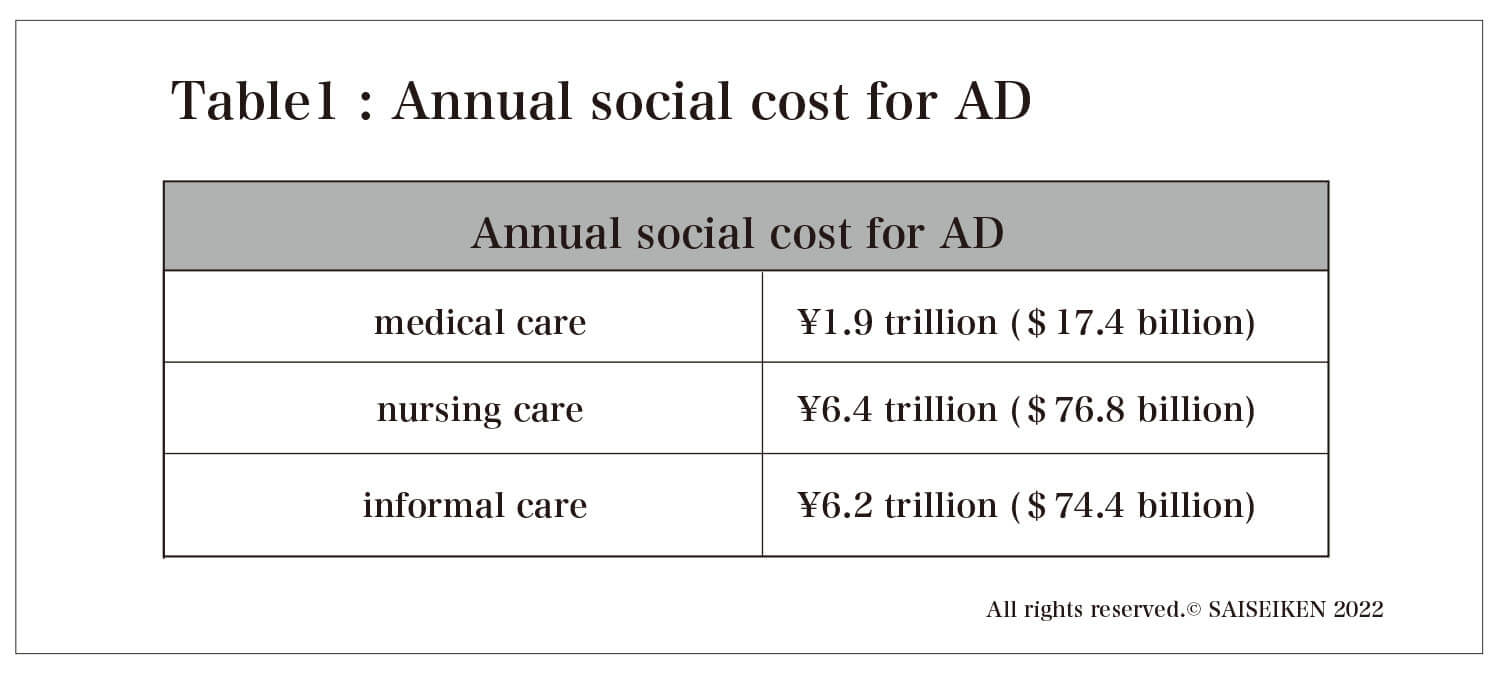
On the other hand, analysis of the social impact of AD has lagged behind, and in Japan, Keio University pioneered the "Estimation of the Social Cost of Dementia" in 2015. According to this estimate, the annual social cost of AD as of 2014 was 14.5 trillion yen. The breakdown of the cost is 1.9 trillion yen for medical care (outpatient and inpatient), 6.4 trillion yen for nursing care (nursing homes and home support), and 6.2 trillion yen for informal care costs (care provided free of charge by family members and others) (Table 1). It should be noted here that the cost of nursing care is more than seven times higher than the cost of pure treatment. In other words, it must be understood that AD is a unique disease in which care is the main focus rather than treatment.
The number of people certified as requiring long-term care (including those requiring support) continues to increase, from 6.55 million in 2018 to an estimated 9.88 million by 2040. The number of people certified as needing nursing care will naturally increase, and the shortage of nursing home staff, which is still a shortage today, will reach 550,000 by 2025, but there is no prospect of securing these personnel. Even if the number of caregivers could be secured by hiring foreign personnel, the overall cost of long-term care will exceed 20 trillion yen by 2040, based on data from Keio University, because the cost of long-term care is proportional to the number of people requiring long-term care. In fact, approximately 20% of the national budget will be spent on AD care alone.
From a different perspective, however, if treatment for dementia is realized, it will not only lead to a decrease in nursing care costs by enabling patients to become independent from a treatment-centered lifestyle, but it will also mean that family members and others freed from nursing care can return to economic activities. As a result, it can be said that new wealth is created as much as the informal care costs for AD patients. Specifically, the average informal care time for one person in need of care is estimated to be 29.97 hours/week, so the annual informal care cost can be calculated to be 3.82 million yen. The same amount of labor productivity would be generated by reducing the number of persons requiring nursing care by one person.
In Europe and the U.S., the fact that AD is deeply linked to the economic activities of the entire country was recognized early on, and research on AD treatment drugs has been promoted as a national strategy.
As a result, many candidate drugs have been developed since 2011. Among them, the leader was a drug for mild dementia (MCI) called "aducanumab" co-developed by Biogen of the U.S. and Eisai of Japan, which was rapidly approved by the U.S. FDA (Food and Drug Administration) on June 7, 2021. It was. However, this drug is strictly a prophylactic drug used before the onset of the disease, not a cure, and a validation study has been ordered after its approval. In other words, the FDA was not convinced of the drug's efficacy based on the clinical trial data published by Biogen (as of July 12, the Acting Commissioner of the FDA requested an investigation into the appropriateness of the approval process).
The development of drugs to treat Alzheimer's disease, not just "aducanumab," continues to be a struggle. In fact, there are no promising new drug candidates, and in fact, since 2018, there have been a series of announcements of development halts, and even giant companies such as Roche (Switzerland) and Pfizer (U.S.) have been forced to abandon development.
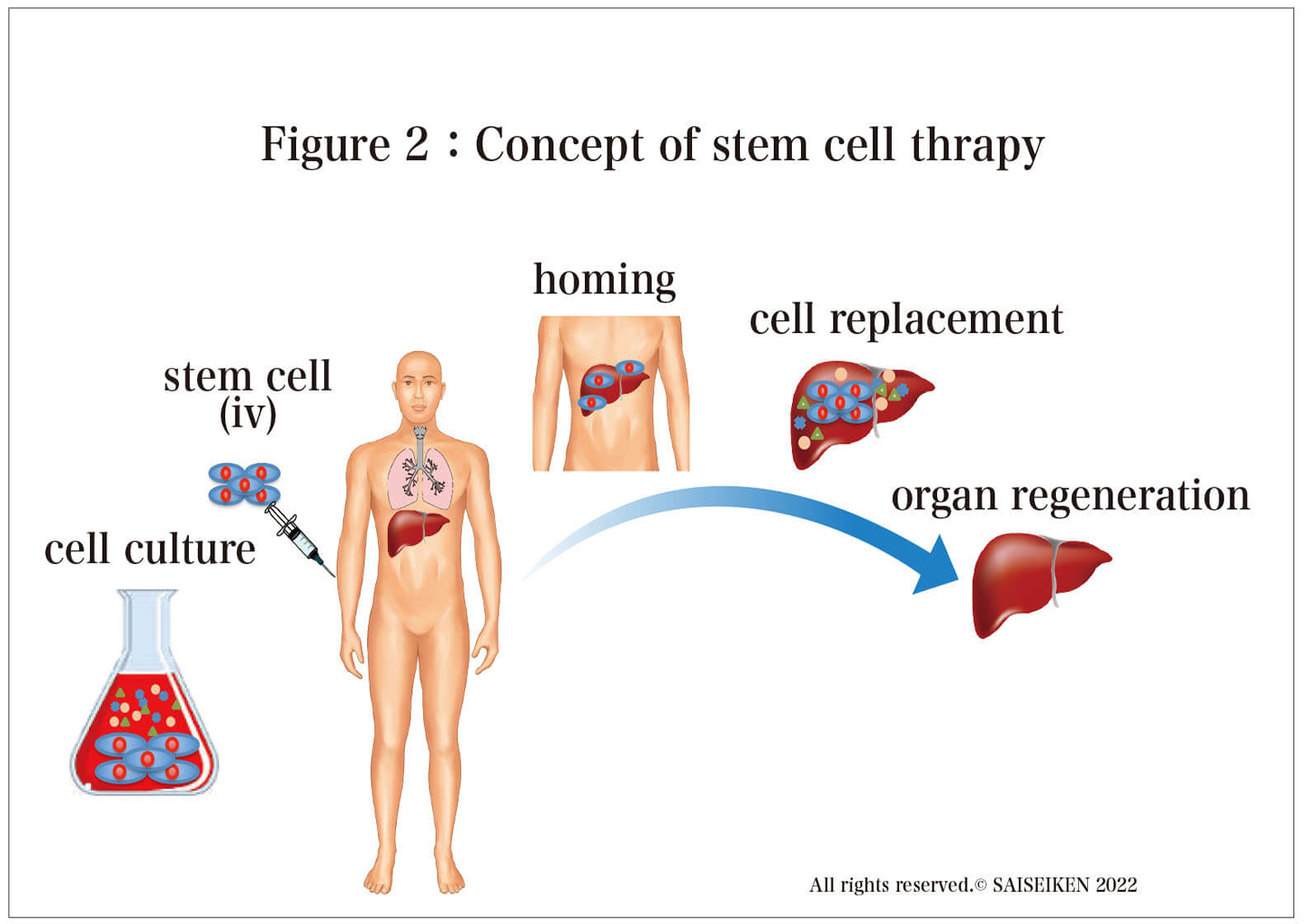
Research on stem cell therapy began in early 2000, and almost all intractable diseases, including AD, have been targeted . Nowadays, stem cell therapy is moving from the basic research stage to clinical trials, and papers published every day from around the world are reporting the promise of stem cell therapy(Figure 2). Especially for intractable diseases such as AD, for which drug treatment is almost hopeless, stem cell therapy is the last remaining fortress. Unlike conventional drugs that remove neurotoxins and protect cells, stem cell therapy is an attractive concept because it can replenish the nerve cells themselves, making it a trump card for the treatment of AD, a disease in which nerve cells are irreversibly lost.
The most well-known stem cell therapy for AD is a project at the University of Miami. Based on the results of animal studies, they are starting a clinical trial in 2019 using umbilical cord-derived allogeneic mesenchymal stem cells (hMSCs).Here is a brief description of the clinical trial.
Patients between the ages of 50 and 85 who have been diagnosed with mild Alzheimer's disease, such as those who score 24 or below on the MMSE test (a test that measures cognitive, spatial, and language abilities on a 30-point scale), will receive four infusions of hMSCs, 100 million cells per infusion (400 million cells in total) (Figure 2). The observation period is 65 weeks, during which time blood tests, various scores, spinal fluid tests, MRI, and PET scans will be conducted to evaluate the effectiveness of the treatment. The final results have not yet been obtained, as the scheduled completion date is September 2022. This is the first ever stem cell therapy for AD, so it has attracted worldwide attention. However, even if this trial is successful and it is proven that AD can be improved by the administration of stem cells, a major problem remains. The cost is an issueLet us simulate what would happen if the same treatment as the University of Miami's stem cell therapy were to be performed in Japan.
Currently, two stem cell drugs, MSCs, are approved in Japan. Let us assume that they are used for other than the intended use in the treatment of AD.
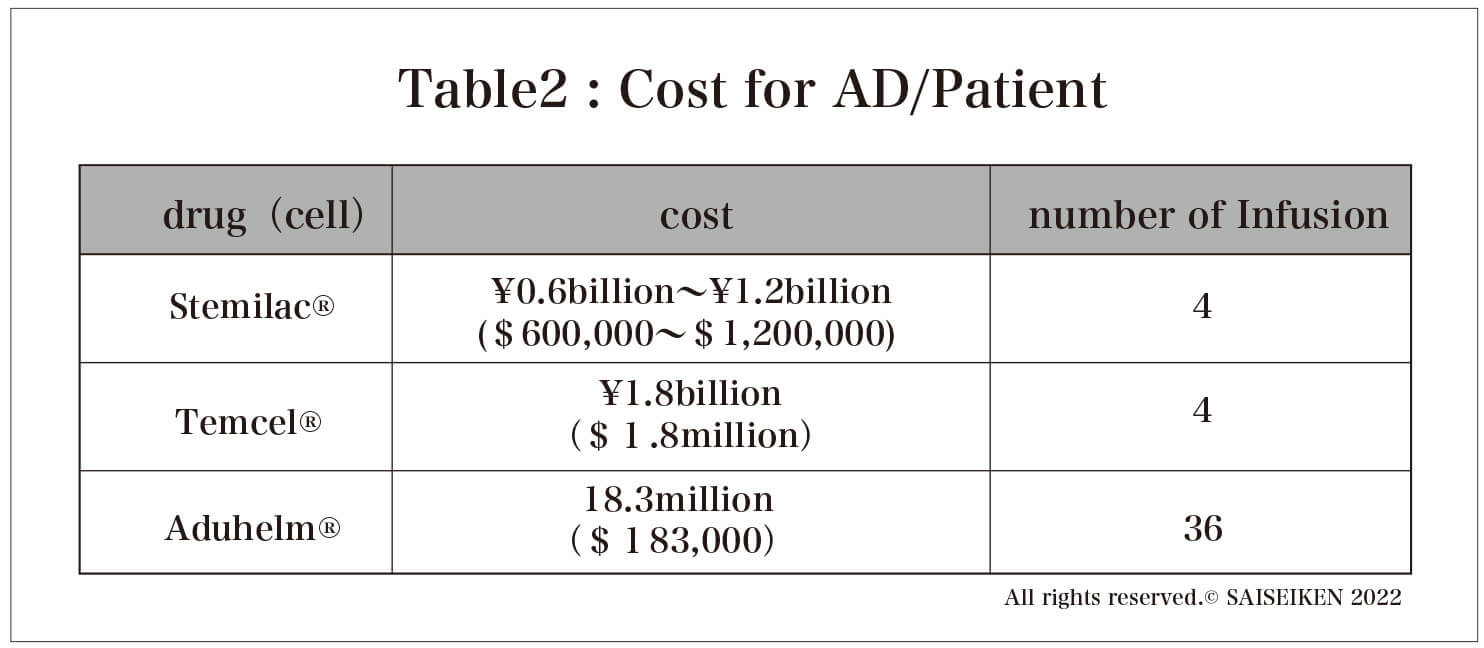
One is the (allogeneic) bone marrow-derived MSC "Temcel " each bag contains 2 million MSCs and is priced at approximately 885,000 yen. If the University of Miami protocol is followed, a total of 400 million MSCs are needed, so the treatment cost is 177 million yen.
The other is " Stemilac ", a stem cell drug approved for spinal cord injury (approved in December 2018), which contains 0.5 to 200 million (autologous) MSCs per bag and costs about 15 million yen per bag, so the treatment cost is between 30 million yen (for 200 million MSCs per pack) and 60 million yen ( (In the case of 0.5 billion cells per bag). However, there was information (Nikkei Biotech) that the cost of culturing the cells was 30 million yen, so the true cost is double that, and if corporate profits are added, the actual treatment cost would be more than 100 million yen per AD patient. In any case, a huge cost burden is inevitable for stem cell therapy for AD(Table 2).
An optimistic counterargument is that if a cure for AD can be developed and patients' health restored, the high cost of home medical care and care in nursing homes can be avoided.
Will it work that well?
The number of AD patients in Japan will be 7.3 million in 10 years.
Even if only 1% of them (73,000 people) receive stem cell therapy, the cost per patient will amount to 7.3 trillion yen if the therapy costs 100 million yen. Who in the world is going to absorb this cost and how?
Regardless of the validity of the science, stem cell therapy has the fatal problem of cost.
There is one solution. Reduce the cost of stem cell production.
If the manufacturing cost can be reduced to 1/100 of the current cost, the cost per patient would be 1 million yen, which is equivalent to the current AD medical cost of 14.5 trillion yen, which is equivalent to the cost of 7.3 million people in Japan. Stem cell therapy for all AD patients would be possible. However, if costs cannot be reduced, stem cell therapy for AD will be a " Pie in the sky ". The time has come to consider not only the scientific arguments about the efficacy and safety of stem cell therapy, but also the economic justification of stem cell therapy.
Conditioned medium therapy" is an evolution of "stem cell therapy.
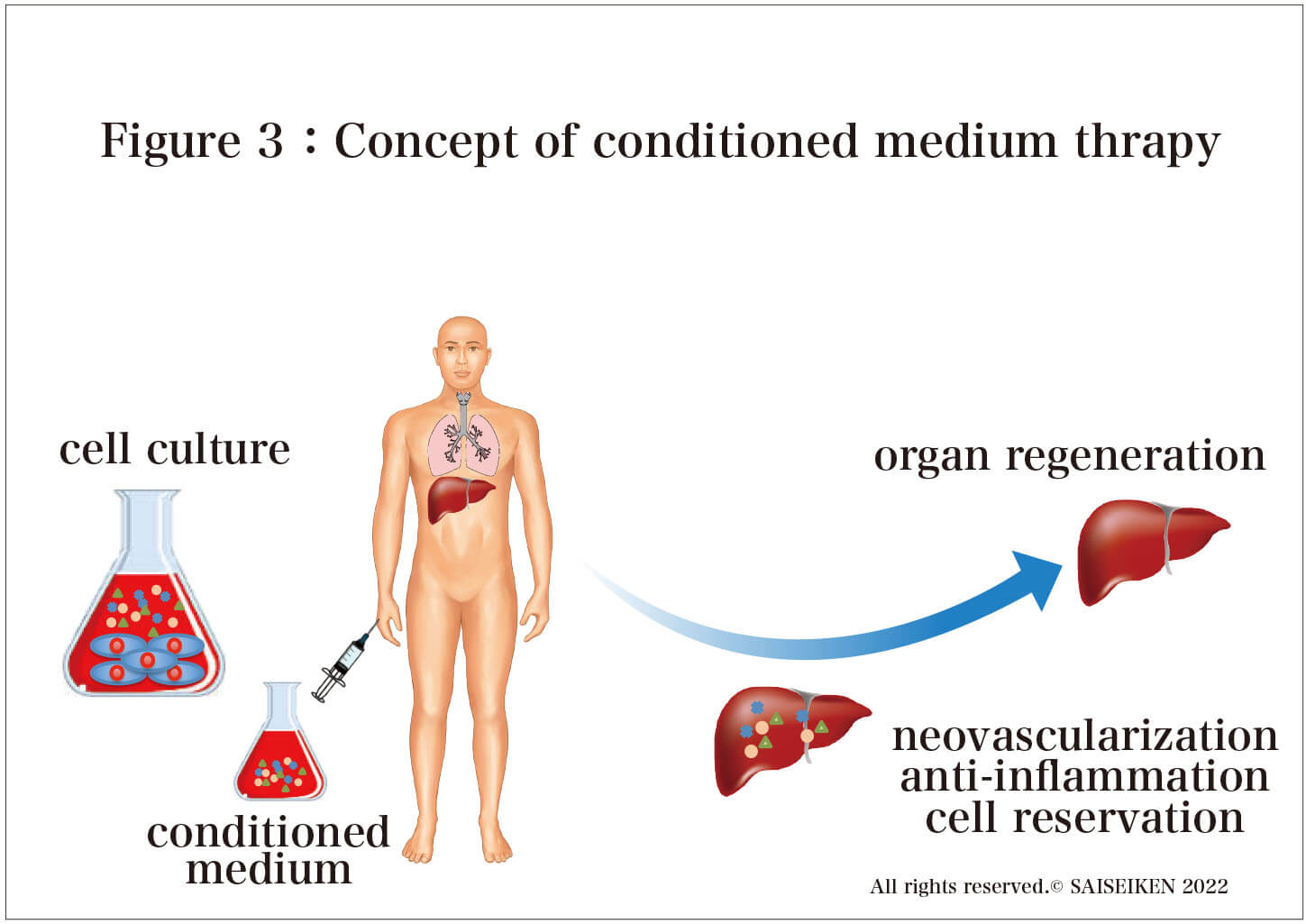
Instead of transplanting stem cells, only the protein produced by stem cells is administered through intravenous infusion or intranasal drip (Figure 3)
The effects of "Conditioned medium therapy" are almost the same as those of "stem cell therapy. However, the cost can be reduced to 1/100. We have launched this website to make people aware of this fact.
If conditioned medium therapy becomes widespread and treatment of AD is realized
"A woman who retired to care for her family."
"A woman who retired to care for her family."
「Times They are a-changin'」
Even with today's advanced medical care, there are still many diseases where patient satisfaction with treatment is low and there is room for improvement. These include cases in which there is no cure for the disease and the patient does not feel that the treatment is sufficiently effective, or cases in which the high cost of treatment places a heavy burden on the patient. Such requests for treatment methods from the patient's point of view are "Unmet medical needs. Therefore, diseases with strong "Unmet medical needs" are the ones that we, medical researcher should place the highest priority on.
According to the American Association of Research Pharmaceutical Manufacturers, Alzheimer's disease (AD) is a prime example of an "Unmet medical need," with the lowest level of patient satisfaction, indicating that current treatments are of little use (Figure 1)。
AD is a disease with a large number of patients and is likely to increase in the future, and its control is becoming an urgent global issue because of the social and economic pressures it places on society. The direct impact of the increase in AD is a dramatic increase in the cost of medical care and nursing care. However, an even more serious impact is that families (often women and young people) are forced to give up work in order to care for AD patients. For the individual, this will mean a setback in life, and for society, it will directly lead to a shortage in the workforce, which will eventually impede the economic growth of our country. Conversely, if a cure for AD were found, it would not only lead to medical success in restoring the health of the individual patient, but also to social benefits such as an increase in the working population and labor productibility .
As a greeting to you , I would like to first explain the social and economic problems of AD, and then introduce the challenges of current AD treatment and the "Conditioned medium therapy" that may break through these challenges.
The International Alzheimer's Association estimates that the number of patients with dementia (AD accounts for more than 70% of the patients) worldwide will be 76 million in 2030 , respectively. It is estimated that the number of people will reach 135 million in 2050. Data for Japan is available in the White Paper on Aging Society published by the Cabinet Office in 2017. According to the report, it is estimated that 7.3 million people will have AD by 2025 and more than 10 million people by 2050, or one out of every four people over 65 years old.
On the other hand, analysis of the social impact of AD has lagged behind, and in Japan, Keio University pioneered the "Estimation of the Social Cost of Dementia" in 2015. According to this estimate, the annual social cost of AD as of 2014 was 14.5 trillion yen. The breakdown of the cost is 1.9 trillion yen for medical care (outpatient and inpatient), 6.4 trillion yen for nursing care (nursing homes and home support), and 6.2 trillion yen for informal care costs (care provided free of charge by family members and others) (Table 1). It should be noted here that the cost of nursing care is more than seven times higher than the cost of pure treatment. In other words, it must be understood that AD is a unique disease in which care is the main focus rather than treatment.
The number of people certified as requiring long-term care (including those requiring support) continues to increase, from 6.55 million in 2018 to an estimated 9.88 million by 2040. The number of people certified as needing nursing care will naturally increase, and the shortage of nursing home staff, which is still a shortage today, will reach 550,000 by 2025, but there is no prospect of securing these personnel. Even if the number of caregivers could be secured by hiring foreign personnel, the overall cost of long-term care will exceed 20 trillion yen by 2040, based on data from Keio University, because the cost of long-term care is proportional to the number of people requiring long-term care. In fact, approximately 20% of the national budget will be spent on AD care alone.
From a different perspective, however, if treatment for dementia is realized, it will not only lead to a decrease in nursing care costs by enabling patients to become independent from a treatment-centered lifestyle, but it will also mean that family members and others freed from nursing care can return to economic activities. As a result, it can be said that new wealth is created as much as the informal care costs for AD patients. Specifically, the average informal care time for one person in need of care is estimated to be 29.97 hours/week, so the annual informal care cost can be calculated to be 3.82 million yen. The same amount of labor productivity would be generated by reducing the number of persons requiring nursing care by one person.
In Europe and the U.S., the fact that AD is deeply linked to the economic activities of the entire country was recognized early on, and research on AD treatment drugs has been promoted as a national strategy.
As a result, many candidate drugs have been developed since 2011. Among them, the leader was a drug for mild dementia (MCI) called "aducanumab" co-developed by Biogen of the U.S. and Eisai of Japan, which was rapidly approved by the U.S. FDA (Food and Drug Administration) on June 7, 2021. It was. However, this drug is strictly a prophylactic drug used before the onset of the disease, not a cure, and a validation study has been ordered after its approval. In other words, the FDA was not convinced of the drug's efficacy based on the clinical trial data published by Biogen (as of July 12, the Acting Commissioner of the FDA requested an investigation into the appropriateness of the approval process).
The development of drugs to treat Alzheimer's disease, not just "aducanumab," continues to be a struggle. In fact, there are no promising new drug candidates, and in fact, since 2018, there have been a series of announcements of development halts, and even giant companies such as Roche (Switzerland) and Pfizer (U.S.) have been forced to abandon development.
Research on stem cell therapy began in early 2000, and almost all intractable diseases, including AD, have been targeted . Nowadays, stem cell therapy is moving from the basic research stage to clinical trials, and papers published every day from around the world are reporting the promise of stem cell therapy(Figure 2). Especially for intractable diseases such as AD, for which drug treatment is almost hopeless, stem cell therapy is the last remaining fortress. Unlike conventional drugs that remove neurotoxins and protect cells, stem cell therapy is an attractive concept because it can replenish the nerve cells themselves, making it a trump card for the treatment of AD, a disease in which nerve cells are irreversibly lost.
The most well-known stem cell therapy for AD is a project at the University of Miami. Based on the results of animal studies, they are starting a clinical trial in 2019 using umbilical cord-derived allogeneic mesenchymal stem cells (hMSCs).Here is a brief description of the clinical trial.
Patients between the ages of 50 and 85 who have been diagnosed with mild Alzheimer's disease, such as those who score 24 or below on the MMSE test (a test that measures cognitive, spatial, and language abilities on a 30-point scale), will receive four infusions of hMSCs, 100 million cells per infusion (400 million cells in total) (Figure 2). The observation period is 65 weeks, during which time blood tests, various scores, spinal fluid tests, MRI, and PET scans will be conducted to evaluate the effectiveness of the treatment. The final results have not yet been obtained, as the scheduled completion date is September 2022. This is the first ever stem cell therapy for AD, so it has attracted worldwide attention. However, even if this trial is successful and it is proven that AD can be improved by the administration of stem cells, a major problem remains. The cost is an issueLet us simulate what would happen if the same treatment as the University of Miami's stem cell therapy were to be performed in Japan.
Currently, two stem cell drugs, MSCs, are approved in Japan. Let us assume that they are used for other than the intended use in the treatment of AD.
One is the (allogeneic) bone marrow-derived MSC "Temcel " each bag contains 2 million MSCs and is priced at approximately 885,000 yen. If the University of Miami protocol is followed, a total of 400 million MSCs are needed, so the treatment cost is 177 million yen.
The other is " Stemilac ", a stem cell drug approved for spinal cord injury (approved in December 2018), which contains 0.5 to 200 million (autologous) MSCs per bag and costs about 15 million yen per bag, so the treatment cost is between 30 million yen (for 200 million MSCs per pack) and 60 million yen ( (In the case of 0.5 billion cells per bag). However, there was information (Nikkei Biotech) that the cost of culturing the cells was 30 million yen, so the true cost is double that, and if corporate profits are added, the actual treatment cost would be more than 100 million yen per AD patient. In any case, a huge cost burden is inevitable for stem cell therapy for AD(Table 2).
An optimistic counterargument is that if a cure for AD can be developed and patients' health restored, the high cost of home medical care and care in nursing homes can be avoided.
Will it work that well?
The number of AD patients in Japan will be 7.3 million in 10 years.
Even if only 1% of them (73,000 people) receive stem cell therapy, the cost per patient will amount to 7.3 trillion yen if the therapy costs 100 million yen. Who in the world is going to absorb this cost and how?
Regardless of the validity of the science, stem cell therapy has the fatal problem of cost.
There is one solution. Reduce the cost of stem cell production.
If the manufacturing cost can be reduced to 1/100 of the current cost, the cost per patient would be 1 million yen, which is equivalent to the current AD medical cost of 14.5 trillion yen, which is equivalent to the cost of 7.3 million people in Japan. Stem cell therapy for all AD patients would be possible. However, if costs cannot be reduced, stem cell therapy for AD will be a " Pie in the sky ". The time has come to consider not only the scientific arguments about the efficacy and safety of stem cell therapy, but also the economic justification of stem cell therapy.
Conditioned medium therapy" is an evolution of "stem cell therapy.
Instead of transplanting stem cells, only the protein produced by stem cells is administered through intravenous infusion or intranasal drip (Figure 3)
The effects of "Conditioned medium therapy" are almost the same as those of "stem cell therapy. However, the cost can be reduced to 1/100. We have launched this website to make people aware of this fact.
If conditioned medium therapy becomes widespread and treatment of AD is realized
"A woman who retired to care for her family."
"A woman who retired to care for her family."Young people who couldn't go to school because of caregiving.
Young people who could not find work because of caregiving.
"Heartbreak and murder due to caregiver exhaustion."
and other social problems may be eliminated. Even if the disease cannot be completely cured, the burden on caregivers will be completely different if the patient's head is strong and the caregiving level is lightened even a little. The treatment of AD is a social problem itself.
Conditioned medium therapy has the power to cure AD and solve these social problems. Bob Dylan, the winner of the 2016 Nobel Prize in Literature, wrote his masterpiece "The times They are a-changin'. Let's think about the "changing times" in the scientific world together with you through this website.

(M.UEDA) Profile
(M.UEDA) Profile
Medical Researcher/Maxillofacial Surgeon
1949 Born in Osaka ,Japan
1978 Graduated from Tokyo Medical and Dental University
1982 Post-graduate School of Medical Science Nagoya University Medicine
1994 Professor of Oral and Maxillofacial Surgery, Nagoya University Graduate School of Medicine
2003 Visiting Professor, The Institute of Medical Science, The University of Tokyo
2015 Professor Emeritus of Nagoya University Japan
1949 Born in Osaka ,Japan
1978 Graduated from Tokyo Medical and Dental University
1982 Post-graduate School of Medical Science Nagoya University Medicine
1994 Professor of Oral and Maxillofacial Surgery, Nagoya University Graduate School of Medicine
2003 Visiting Professor, The Institute of Medical Science, The University of Tokyo
2015 Professor Emeritus of Nagoya University Japan
History
History
Over seas
2020 Special Advisor Clinical Trial, The University of Bergen Hospital
"Treatment of multiple sclerosis using mesenchymal stem cells."
Awards
2010 Best Professor Award, Nagoya University Graduate School of Medicine
2016 The Jonson & Jonson Award of the Japanese Society of Regenerative Medicine
The first in human
2011 Stroke
2013 Diabetes
2014 Atopic dermatitis
2015 Alzheimer’s Disease
2016 Knee osteoarthritis / rheumatoid arthritis of the hand and fingers
2021 Amyotrophic lateral sclerosis (ALS) / Interstitial pneumonia / Diabetic nephropathy

